Translational Research Partnership Awards Program - Phase I

Based on what it learned from the pilot program, the Foundation launched the Translational Research Program (TP) in 2004. The Foundation began a rigorous four-step process to select its partners. Of the 100 eligible BME departments, nine were selected.
Building a Formal Translational Research Program in Academia
Based on the tools developed and lessons learned during the Translational Research Partnership Program, described below, other universities should be able to implement formal Translational Research programs.
The decision criteria for selection of the partner schools included opportunity for impact, a committed school of medicine, a well-resourced Office of Technology Transfer (OTT), a commitment to expand the BME department, creation of the Coulter Program Director (CPD) position, and assembly of an Oversight Committee (OC). The chemistry for partnership between the university and the Foundation was essential.
The mix of schools was diverse by design. There were large and small universities, public and private institutions, urban and rural locations, new and established BME departments, as well as large and small research budgets. The Coulter Program had additional requirements for the OTT. In many US universities, the OTT was insufficiently funded and focused on the cost of managing its intellectual property (IP). Common practice was to patent and out-license IP, but most offices lacked the resources to proactively seek industry partnerships and commercialization opportunities. The Coulter Program required active participation of OTT team members throughout every project’s life cycle. The common practice of engineers and scientists was to present discoveries to OTT for patenting without regard for their commercial viability. This practice was transformed to one that is dynamic and requires active participation for commercial analysis, industry validation and identification of potential sources of follow on funding. The program validated the critical role of OTT.
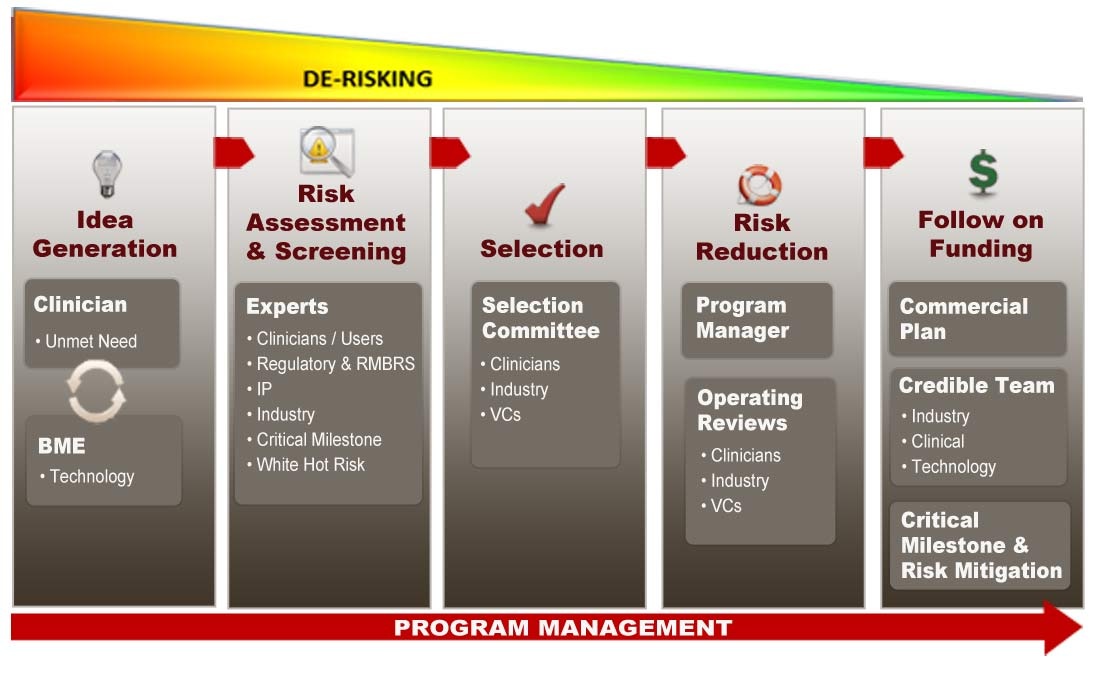
At the core of the Coulter Commercialization Process is the desire to identify ideas, developed from interactions between clinicians and biomedical engineers, for solutions to unmet clinical needs. The projects are managed to the point where risk is reduced enough to attract professional follow-on funding, the metric of success. Although follow-on funding and commercialization are not the primary goals of the Program, they are critical steps to bringing innovations to patients.
The expected outcomes for the Program are the following. The partner universities would become:
- Powerhouses of translational research
- Preferred partners for industry and venture capital
- Economic engines for their community
The Foundation and the universities worked for almost a year to adapt the product development practices of industry for use in academia. In what came to be known as the “Coulter Commercialization Process”, it is a guide to accelerate academic innovations using business practices that can be implemented by any university. Although formulaic in the identification of key success elements, each program has the freedom to operate within its own ecosystem and leverage their competencies for implementation. In addition, job process requirements for the four strategic stakeholders were created. At the start of this five-year program, success meant attracting follow-on funding for projects. The success metric was defined as the number of projects that were licensed to professional sources of funding.
Coulter TR Partner Universities, Phase I
- Boston University, Boston, MA
- Case Western Reserve University, Cleveland, OH
- Drexel University, Philadelphia, PA
- Duke University, Durham, NC
- Georgia Institute of Technology / Emory University, Atlanta, GA
- Stanford University, Stanford, CA
- University of Michigan, Ann Arbor, MI
- University of Virginia, Charlottesville, VA
- University of Washington, Seattle WA
- University of Wisconsin, Madison, WI
By the end of the third year, all stakeholders understood their roles and responsibilities. Successful projects started to attract external funds and this steadily accelerated through the fifth year. The OC recognized which projects should be funded or stopped. The OTT developed networks with industry, VCs and other sources of follow-on funding. The project teams together with the OTT, OC, and CPDs were able to better define the proof-of-concept or “Killer Experiment” based on expert feedback. The Killer Experiment, a significant step in the project de-risking process, is defined as an experiment for which the outcome either increases confidence toward further development of the project or recommends the project be “killed” (stopped).
As of March 31, 2013, with an investment of $70MM, about 280 projects had received at least one year of funding. Thirty-one were licensed to established medical companies. Of 60 start-ups, 55 received VC funding in excess of $900MM and another twenty were seeking funding. It is important to note that most of the universities were able to achieve a project success rate in excess of 30.7 percent. Neither geographic location nor research budget were relevant predictors of success. An unanticipated program result was the generation of more than $500MM in additional government funding. While these early results were striking, the program continues to succeed, with projects attracting follow-on funding and seven of the original ten universities have committed to continue this program into perpetuity.